H 66 1034 Kgm2s h 66 10 34 K g m 2 s This formula for l l is called the de Broglie relation and l l is called the de Broglie wavelength of the electron. N number of orbit. Note that when considering electromagnetic radiation such as light the quantity speed is.
6 1 0 1 9 1 0 0 9 1 0 3 1 6 1 0 6 m s.
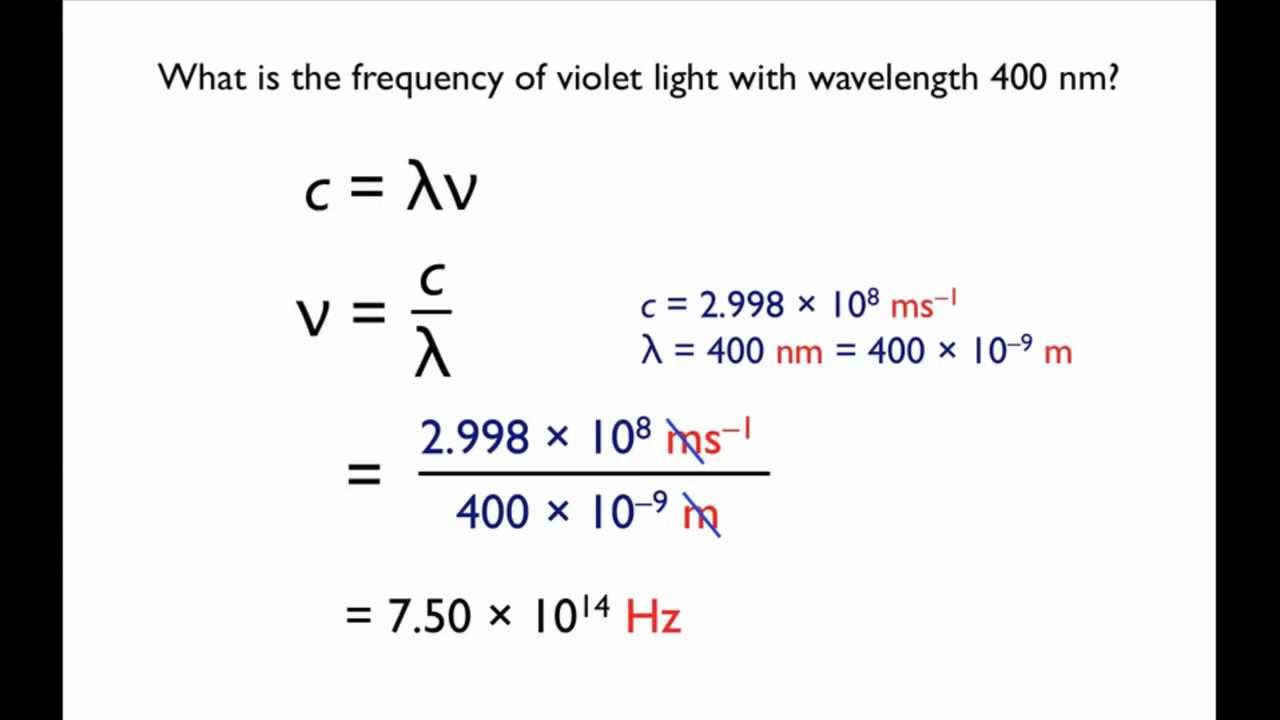
The formulas are shown together with other useful information and examples to try. E hc l where. F frequency in Hertz Hz 1 sec l wavelength in meters m c the speed of light 299792458 m s E energy in electron Volts eV h Planks constant 6626068 10-34 m 2 kg s. Where the photon energy was multiplied with the electronic charge to convert the energy in Joule rather than electron Volt.