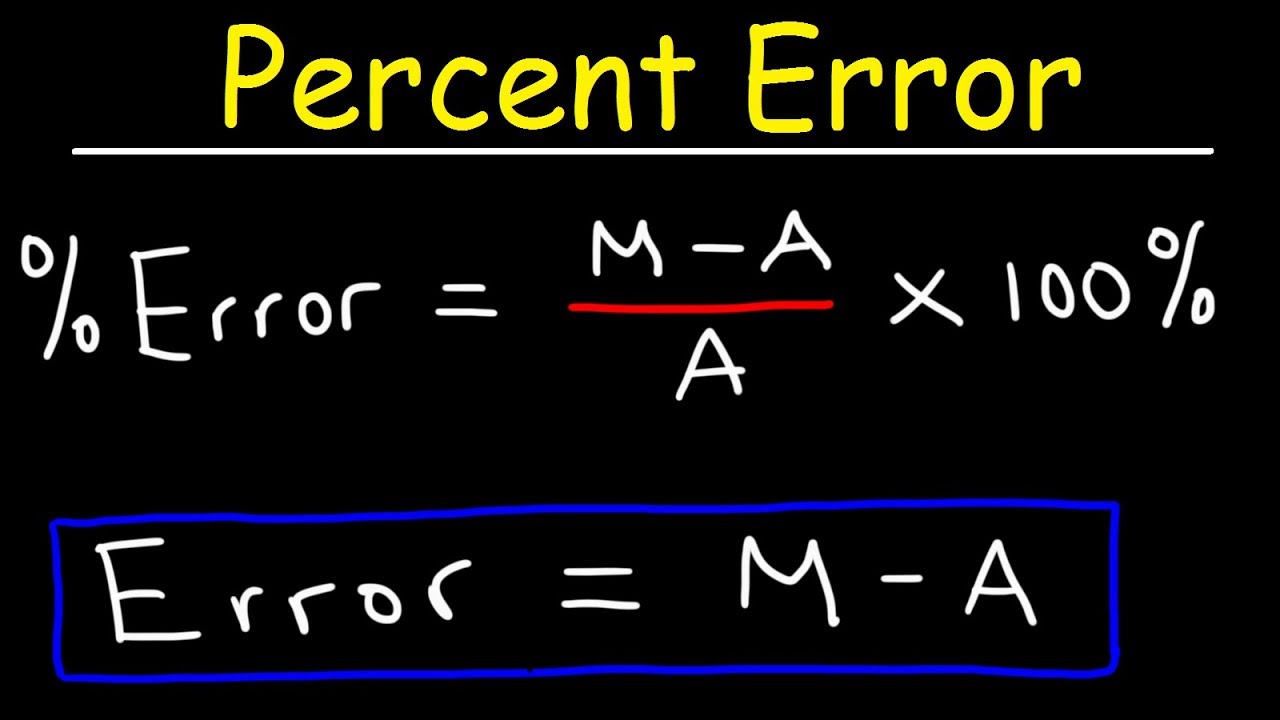
The theoretical yield of alum was then calculated assuming that aluminum was the limiting reactant and that the foil was 100 aluminum and was then used to calculate the percent yield. You need to begin with a balanced chemical equation and define the limiting reactant. So 1g Al gives 25827g alum.
You determine percent yield of a chemical reaction with the following formula.
PREPARATION OF POTASSIUM ALUMINUM SULFATE KAISO212 H2O ALUM DATA AND RESULTS 198 19915 Mass of 250-mL beaker Mass of 250-mL beaker and aluminum 115 4559 Mass of aluminum used watch glass Mass of clean dry 250mbeeer 6006 Mass of 50an bealkenand alum 1447 Mass of alum obtained Calculate the theoretical yield of the alum based on. The percent yield of alum is calculated based on the starting quantity of aluminum. How much product should the experiment have produced if the limiting reagent was totally and efficiently consumed. The theoretical yield is a term used in chemistry to describe the maximum amount of product that you expect a chemical reaction could create.